There has been a flurry of COVID-19 vaccine news over the past few weeks. With booster shots now available for many North Carolinians and a new supply of vaccines for children 5-11, our reporters Will Michaels and Laura Pellicer tackle the big questions for residents looking to protect themselves and their families.
There's only one vaccine so far that's available to kids 5 to 11 and 12 to 17, and that's the Pfizer vaccine. Here in North Carolina, kids and teens 12 and up have been able to get the Pfizer vaccine since early summer. So far, about 350,000 kids in that age group have gotten at least one dose of the Pfizer vaccine.
A couple of weekends ago, the state started receiving the Pfizer vaccine that is specifically dosed for 5 to 11-year-olds, and about 25,000 kids in that age group have received a dose so far. That number may seem low, but it's important to consider the time it takes for parents to schedule the shot and to bring their kids to a vaccine site.
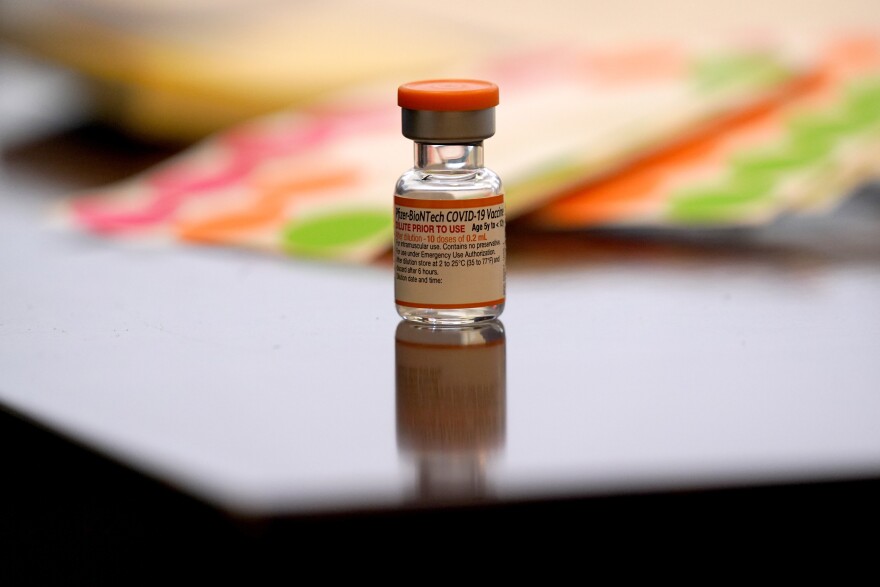
It's interesting to note that the packaging on the boxes and vials for the child-dosed Pfizer vaccines are different than for adults. The lowest dose for the youngest kids has a bright orange cap on the vial and comes in a bright orange box. The packaging is designed to prevent errors and make sure that the youngest kids are getting the right dosage.
Moderna and Johnson & Johnson are lagging behind when it comes to clinical testing and emergency approval for children. Moderna started recruiting participants for the under-12 category for their clinical trials back in March.
Last month, the FDA said that the agency would need more time to assess the Moderna vaccine for teens 12 to 17. The FDA wants to assess the risk of myocarditis, an inflammation of the heart muscle, after vaccination. This reaction from the vaccines is very rare. Moderna should wrap up a review of that age group by January.
A spokesperson Johnson & Johnson told WUNC that the company has started Phase 3 clinical trial testing for ages 12 to 17. So everyone's a little behind Pfizer at this point.
Testing and approving COVID-19 vaccines for kids is a rigorous process in the U.S. First, the vaccine manufacturers test the vaccine in clinical trials. The company gathers the safety and efficacy data and sends that data to government health agencies. Then an advisory panel at the FDA reviews that information and votes on whether the benefits outweigh any risks. Then the FDA — the agency itself — considers the panel's vote. The agency decides whether to extend emergency authorization. Then it goes to the CDC advisory panel, after that, the CDC director decides whether to support the decision of the CDC advisory panel.
So along the way, there are independent scientists, experts, government officials who review the data and provide a "check" on each other's work. This piece from NPR gives a helpful timeline of what this process looks like for the Pfizer vaccine dose for young kids.
This is one of those questions that can send you down a Google rabbit hole, so let's have an expert weigh in. Dr. David Wohl, a physician at UNC-Chapel Hill in the Division of Infectious Diseases, told us that he would lean towards getting the vaccine now (before the child is 12), but with some caveats.
Clinics are supposed to adhere to the age of the child at the time of vaccination. In other words, if your kid is 11 years and 11 months old, in most cases they would receive the under-12 dosage of the Pfizer vaccine. But Dr. Wohl says there are some personal factors that each family might consider, and one is weight. If your child is 11, going on 12, but they weigh more than 40 kilos or 80 pounds, they might benefit from a higher dose. In that case, the best move is to consult directly with your pediatrician or family doctor about the dosage.

Let's take this manufacturer by manufacturer because it's a little bit different for each. Right now, the recommendations are if you had the single-dose Johnson & Johnson vaccine, and it's been at least two months since you had that dose, that anyone at any age could go ahead and get a booster shot.
For those who had the Pfizer or Moderna double doses, if it's been at least six months since you had those doses, and you are over 65 or have underlying medical conditions or are at a higher risk of exposure, you should go ahead and get a booster shot.
And there is some preliminary data that suggests that if you had that Johnson & Johnson vaccine, that it might be more effective for you to get a booster shot that is an mRNA booster — that would either be the Pfizer or Moderna shots.
Breakthrough infections are verified COVID-19 infections that happen after you are fully vaccinated. If you have a breakthrough infection, it will trigger a significant antibody response, which in turn heightens your immunity. According to the CDC, each person's immune response to a breakthrough case is a little different. A COVID vaccine has a more reliable, consistent, predictable response. Multiple studies have shown that giving a dose to people who have been previously infected with COVID-19 still enhances their immune response.
With that cleared up, let's talk about the timeline of when to get a booster. The CDC doesn't offer a specific timeline in these cases. They simply say if you have had a breakthrough case, you should until that acute infection is over and your symptoms are gone.
Infectious disease specialist Dr. Wohl says it might serve you better to wait longer if you've had a breakthrough case because your immune response was enhanced to a certain degree after that infection. He says if you've had a breakthrough case after being fully vaccinated, it's akin to having a booster shot. So in short, there is no rush if you've had a breakthrough case.
Dr. Wohl says a booster shot is most important for immunocompromised people who might have more severe infections if they catch COVID-19, or who may not mount the same kind of immune response as someone who isn't immunocompromised.
Dr. Wohl says he doesn't expect boosters to be needed every six months. But his sense, as someone who has helped lead research and clinical trials for COVID vaccines, is that we may need to get a shot every year or year and a half, much like the flu shot. This timeline may change if another significant variant appears or if the case count that is declining right now goes back up.
There's also some active research into monoclonal antibody treatments, which could be an alternative to booster shots, especially for those immunocompromised individuals. Ultimately though, there isn't a clear, firm answer to this question of how often we'll need boosters — even though it's the biggest question many folks have right now.
Copyright 2021 North Carolina Public Radio